

植物生态学报 ›› 2005, Vol. 29 ›› Issue (6): 910-917.DOI: 10.17521/cjpe.2005.0122
收稿日期:2004-11-01
接受日期:2005-04-07
出版日期:2005-11-01
发布日期:2005-09-30
通讯作者:
刘颖慧
作者简介:*E-mail:lyh@ires.cn基金资助:
JIA Hai-Kun, LIU Ying-Hui*( ), XU Xia, WANG Kun, GAO Qiong
), XU Xia, WANG Kun, GAO Qiong
Received:2004-11-01
Accepted:2005-04-07
Online:2005-11-01
Published:2005-09-30
Contact:
LIU Ying-Hui
摘要:
在皇甫川流域,随着林草覆盖度的增加,植被与水的矛盾日益突出,其中一个重要的问题就是植被密度与土壤水分之间的矛盾。土壤水分的降低影响了植被的生长,甚至导致了部分植被的死亡,因此对土壤水分与植被密度之间的关系进行研究非常重要,有助于合理造林密度的确定。在已有研究及实验观测的基础上,建立了柠条(Caragana intermedia)林地土壤水分动态模拟模型,模型考虑了主要的土壤、植物过程,包括土壤性状、降雨入渗、植物蒸腾、地表蒸发等;模拟了从1971至2000年,30年里各种立地条件(不同盖度、坡向和坡度)下的柠条林地土壤水分、蒸腾和蒸发等的日动态过程。通过比较不同立地条件下的土壤水分动态,研究了皇甫川流域典型柠条林地土壤水分与植被盖度、坡向和坡度之间的关系,并得出了它们之间的关系式。由得到的平地上柠条的适宜密度,同时结合上述关系式,得出了不同坡度、坡向的适宜密度。坡度小于10°时,适宜造林密度对坡度反应敏感,在10°~30°时,适宜盖度对坡度反应不敏感。对于小于10°的坡地,植被建设时要特别注意设计合理的植被密度。
贾海坤, 刘颖慧, 徐霞, 王昆, 高琼. 皇甫川流域柠条林地水分动态模拟——坡度、坡向、植被密度与土壤水分的关系. 植物生态学报, 2005, 29(6): 910-917. DOI: 10.17521/cjpe.2005.0122
JIA Hai-Kun, LIU Ying-Hui, XU Xia, WANG Kun, GAO Qiong. SIMULATION OF SOIL WATER DYNAMICS IN A CARAGANA INTERMEDIA WOODLAND IN HUANGFUCHUAN WATERSHED: RELATIONSHIPS AMONG SLOPE, ASPECT, PLANT DENSITY AND SOIL WATER CONTENT. Chinese Journal of Plant Ecology, 2005, 29(6): 910-917. DOI: 10.17521/cjpe.2005.0122
| 特征指标 Character indexes | 数值 Value | |||||
|---|---|---|---|---|---|---|
| 砂粒含量 Sand-grain fraction | 82.16% | |||||
| 粉粒含量 Silt fraction | 6.78% | |||||
| 黏粒含量 Clay fraction | 11.06% | |||||
| 田间持水量 Field moisture capacity | 0.19 m3·m-3 | |||||
| 剩余含水量 Residual moisture content | 0.025 m3·m-3 | |||||
| 饱和含水量 Saturation moisture content | 0.37 m3·m-3 | |||||
| 萎蔫含水量 Wilting point moisture | 0.027 m3·m-3 | |||||
| 饱和导水率 Saturated hydraulic conductivity | 10 cm·h-1 | |||||
| 容重 Bulk density | 1.44 g3·cm-3 | |||||
表1 土壤特征指标
Table 1 Soil character indexes
| 特征指标 Character indexes | 数值 Value | |||||
|---|---|---|---|---|---|---|
| 砂粒含量 Sand-grain fraction | 82.16% | |||||
| 粉粒含量 Silt fraction | 6.78% | |||||
| 黏粒含量 Clay fraction | 11.06% | |||||
| 田间持水量 Field moisture capacity | 0.19 m3·m-3 | |||||
| 剩余含水量 Residual moisture content | 0.025 m3·m-3 | |||||
| 饱和含水量 Saturation moisture content | 0.37 m3·m-3 | |||||
| 萎蔫含水量 Wilting point moisture | 0.027 m3·m-3 | |||||
| 饱和导水率 Saturated hydraulic conductivity | 10 cm·h-1 | |||||
| 容重 Bulk density | 1.44 g3·cm-3 | |||||
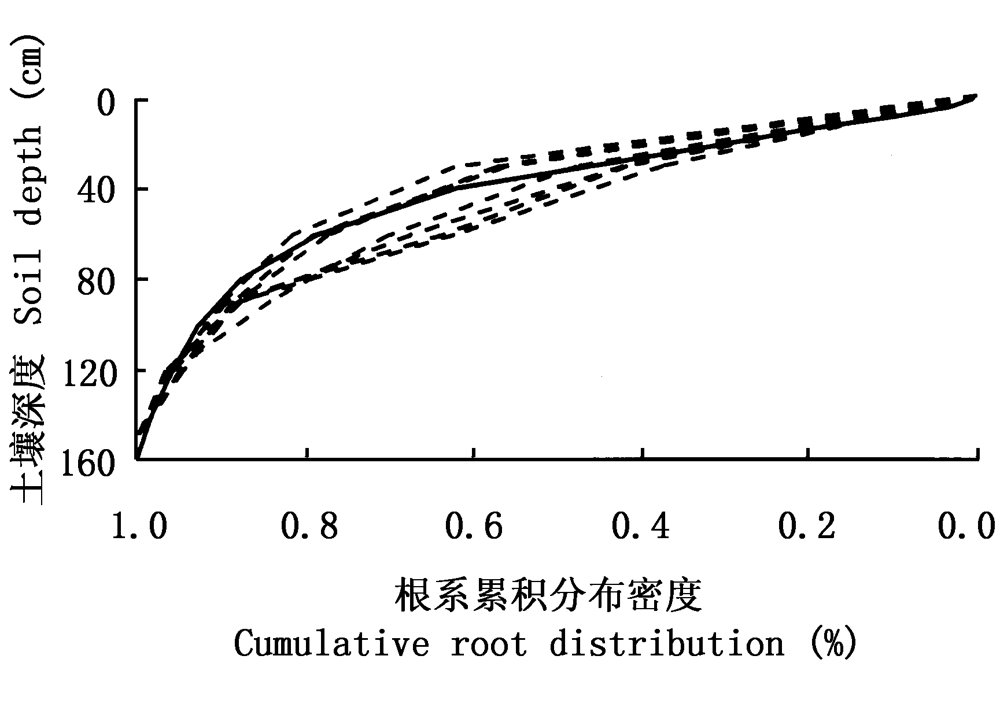
图2 柠条累计根系分布 虚线表示实际测量的根系分布,实线为拟合得到的根系分布函数
Fig.2 Cumulative root distributions of Caragana intermedia The dashed for measured data, the real line for fitted
| 坡度 Slope | 0° | 45° | 90° | 135° | 180° |
|---|---|---|---|---|---|
| 土壤水分与坡度的关系 Soil water content versus slope | ↗ | ↗ | ∩ | ∪ | ↘ |
表2 不同坡向上坡度与土壤水分的关系
Table 2 Types of function between soil water content and slope of different aspect
| 坡度 Slope | 0° | 45° | 90° | 135° | 180° |
|---|---|---|---|---|---|
| 土壤水分与坡度的关系 Soil water content versus slope | ↗ | ↗ | ∩ | ∪ | ↘ |
| [1] | Allen RG (1986). A penman for all seasons. Journal of Irrigation and Drainage Engineering, 112,348-368. |
| [2] | Cui XY (崔骁勇), Chen ZZ (陈佐忠), Du ZC (杜占池) (2001). Study on light- and water- use characteristics of main plants in semiarid steppe. Acta Prataculturae Sinica (草业学报), 10(2),14-21. (in Chinese with English abstract) |
| [3] | Fu BP (傅抱璞), Weng DM (翁笃明), Yu JM (虞静明) (1994). Microclimate (小气候学). China Meteorological Press, Beijing,197-227. (in Chinese) |
| [4] | Fu BJ (傅伯杰) (1991). Study on land assessment in the Loess Plateau of Northern Shaanxi Province. Journal of Soil and Water Conservation (水土保持学报), 5,1-7. (in Chinese with English abstract) |
| [5] | Gao Q, Zhao P, Zeng X, Cai X, Shen W (2002). A model of stomatal conductance to quantify the relationship between leaf transpiration, microclimate and soil water stress. Plant, Cell and Environment, 5,1373-1381. |
| [6] | Gao QZ (高清竹), Yang J (杨吉力), Li GQ (李国强), Jin ZP (金争平), Wang ZW (王正文) (2000). A study on the water regime of Thymus serpyllum community in Huangfuchuan watershed. Acta Agrestia Sinica (中国草地), 5,23-27. (in Chinese with English abstract) |
| [7] | Gao QZ (高清竹) (2003). Land Use Security Pattern for Farming-pastoral Zone of North China, a Case Study at Changchuan Watershed (农牧交错带长川流域土地利用安全格局研究). Ph. D. dissertation, Beijing Normal University, Beijing,19-51. (in Chinese with English abstract) |
| [8] | Jiang DS (蒋定生) (1997). Soil and Water Loss and Its' Management Patterns in the Loess Plateau (黄土高原水土流失与治理模式). China Water Power Press, Beijing,106-122. (in Chinese) |
| [9] | Jin ZP (金争平), Shi PJ (史培军), Hou FC (侯福昌), Zhao HX (赵焕勋) (1992). Soil Erosion System Models and Management Patterns in Huangfuchuan Watershed (黄河皇甫川流域土壤侵蚀系统模型和治理模式). China Ocean Press, Beijing,234-242. (in Chinese) |
| [10] | Jin ZP (金争平), Miao ZY (苗宗义), Wang ZW (王正文), Yan ZQ (阎占卿), Yang J (杨吉力), Li LY (李立业), Fu FL (付福林), Jia ZB (贾志斌), Chai JH (柴建华), Han XS (韩学士), Lin F (蔺丰) (2003). Study on Soil and Water Conservation and the Development of Farming and Grazing in Pishayan Area (砒砂岩区水土保持与农牧业发展研究). Yellow River Water Resources Press, Zhengzhou,48-63. (in Chinese) |
| [11] | Liu JD (刘建栋), Fu BP (傅抱璞), Yu Q (于强) (1998). The study on Penman-Monteith model taking account of the factors of the environment used for water transfer calculation. Journal of Nanjing University (Natural Scinece Edition) (南京大学学报(自然科学版)), 34,359-364. (in Chinese with English abstract) |
| [12] | Liu XZ (刘贤赵), Kang SZ (康绍忠) (1998). An introduction to models of rainfall interception by forest canopy. Journal of Northwest Forestry College (西北林学院学报), 13,26-30. (in Chinese with English abstract) |
| [13] | Luo TX, Neilson RP, Tian H, Vørøsmarty CJ, Zhu H, Liu S (2002). A model for seasonality and distribution of leaf area index of forests and its application to China. Journal of Vegetation Science, 13,817-830. |
| [14] | Miao ZY (苗宗义) (1992). The Comprehensive Managements on Soil and Water Loss: Study on the Developmental Experiments of Agriculture, Forestry and Animal (黄土高原综合治理皇甫川流域水土流失综合治理农林牧全面发展试验研究). China Agricultural Science and Technology Press, Beijing,12-42. (in Chinese) |
| [15] | Niu XW (牛西午) (2003). Studies on Caragana (柠条研究). Science Press, Beijing,48-49. (in Chinese) |
| [16] | Nouvellon Y, Rambal S, Lo Seen D, Moran MS, Lhomme JP, Bèguè A, Chebouni AG, Kerr Y (2000). Modeling of daily fluxes of water and carbon from shortgrass steppes. Agricultural and Forest Meteorology, 100,137-153. |
| [17] | Rawls WJ, Brakensiek DL (1985). Prediction of soil water properties for hydrologic modeling. In: Jones E, Ward TJ eds. Watershed Management in the Eighties. ASCE, New York,293-299. |
| [18] | Rawls WJ, Brakensiel DL, Saxton KE (1982). Estimation of soil water properties. Transactions, American Society of Agricultural Engineers, 25,1316-1330. |
| [19] | Richard GA, Luis SP, Dirk R, Martin S (1998). Crop evapotranspiration - Guidelines for computing crop water requirements - FAO Irrigation and drainage paper 56, Available via DIALOG. http://www.fao.org/docrep/X0490E/X0490E00.htm. |
| [20] | Running SW, Coughlan JC (1988). A general model of forest ecosystem processes for regional applications. I. Hydrologic balance, canopy gas exchange and primary production processes. Ecological Modelling, 42,125-154. |
| [21] |
Ryel RJ, Caldwell MM, Yoder CK, Or D, Leffler AJ (2002). Hydraulic redistribution in a stand of Artemisia tridentata: evaluation of benefits to transpiration assessed with a simulation model. Oecologia, 130,173-184.
DOI URL PMID |
| [22] | Schennk HJ, Robert BJ (2002). The global biogeography of roots. Ecological Monographs, 72,311-328. |
| [23] | Wang J (王军), Fu BJ (傅伯杰), Jiang XP (蒋小平) (2002). Review on research of soil moisture heterogeneity. Research of Soil and Water Conservation (水土保持研究), 9(1):1-5. (in Chinese with English abstract) |
| [24] | Yang J (杨吉力), Gao QZ (高清竹), Li GQ (李国强), He LH (何立环), Jin ZP (金争平), Wang ZW (王正文) (2002). A study on the water ecology of dominant artificial shrubs in Huangfuchuan watershed. Journal of Natural Resources (自然资源学报), 17,87-94. (in Chinese with English abstract) |
| [25] | Yang J (杨吉力), Song BY (宋炳煜), Piao SJ (朴顺姬), Tong C (仝川), Gao QZ (高清竹) (2003) Experimental study on ecological use of water of a small catchment in Huangfuchuan area. Journal of Natural Resources (自然资源学报), 18,513-521. (in Chinese with English abstract) |
| [26] | Yang WZ (杨文治), Yu CZ (余存祖) (1992). Regional Management and Evaluation in the Loess Plateau (黄土高原区域治理与评价). Science Press, Beijing,190-297. (in Chinese) |
| [27] | Yi CX (仪垂祥), Liu KY (刘开瑜), Zhou T (周涛) (1996). Research on a formula of rainfall interception by vegetation. Journal of Soil Erosion and Soil and Water Conservation (土壤侵蚀与水土保持学报), 2,47-49. (in Chinese with English abstract) |
| [28] | Zhang GH (张光辉), Liang YM (梁一民) (1995). The seasonal change of artificial grassland coverage and its' soil and water conservation benefit in loess hilly region. Bulletin of Soil and Water Conservation (水土保持通报), 15(2),38-43. (in Chinese with English abstract) |
| [29] | Zhang GH (张光辉), Liang YM (梁一民) (1996). A summary of impact of vegetation coverage on soil and water conservation benefit. Research of Soil and Water Conservation (水土保持研究), 3(2),104-110. (in Chinese with English abstract) |
| [30] | Zhou YH (周允华), Xiang YQ (项月琴), Luan LK (栾禄凯) (1996). Climatological estimationof quantum flux densities. Acta Meteorologica Sinica (气象学报), 54,447-455. (in Chinese with English abstract) |
| [31] | Zhu ZC (朱志诚) (1993). The main characteristics of the vegetation and its impact on the soil essence in the Loess Plateau of Northern Shaanxi province. Acta Phytoecologica et Geobotanica Sinica (植物生态学与地植物学学报), 17,280-286. (in Chinese with English abstract) |
| [1] | 刘婧, 缑倩倩, 王国华, 赵峰侠. 晋西北丘陵风沙区柠条锦鸡儿叶片与土壤生态化学计量特征[J]. 植物生态学报, 2023, 47(4): 546-558. |
| [2] | 吕婷, 赵西宁, 高晓东, 潘燕辉. 黄土丘陵区典型天然灌丛和人工灌丛优势植物土壤水分利用策略[J]. 植物生态学报, 2017, 41(2): 175-185. |
| [3] | 荐圣淇, 赵传燕, 方书敏, 余凯, 马文瑛. 黄土高原丘陵沟壑区柠条与沙棘冠层的持水能力[J]. 植物生态学报, 2013, 37(1): 45-51. |
| [4] | 陈洁, 汤亮, 刘小军, 曹卫星, 朱艳. 基于植株碳流的水稻籽粒淀粉积累模拟模型[J]. 植物生态学报, 2011, 35(4): 431-440. |
| [5] | 鲍芳, 周广胜. 中国草原土壤呼吸作用研究进展[J]. 植物生态学报, 2010, 34(6): 713-726. |
| [6] | 荀俊杰, 李俊英, 陈建文, 史建伟, 王孟本. 幼龄柠条细根现存量与环境因子的关系[J]. 植物生态学报, 2009, 33(4): 764-771. |
| [7] | 汤亮, 朱艳, 曹卫星. 油菜绿色面积指数动态模拟模型[J]. 植物生态学报, 2007, 31(5): 897-902. |
| [8] | 杨佩国, 李保国, 吕贻忠, 吴绍洪, 李静. 考考赖沟流域植被生产力模拟[J]. 植物生态学报, 2007, 31(5): 787-793. |
| [9] | 张文菊, 童成立, 刘守龙, 宋长春, 吴金水. 三江平原湿地小叶章生产力模拟模型[J]. 植物生态学报, 2006, 30(5): 844-851. |
| [10] | 方向文, 王万鹏, 何小琴, 王刚. 扰动环境中不同刈割方式对柠条营养生长补偿的影响[J]. 植物生态学报, 2006, 30(5): 810-816. |
| [11] | 张志山, 李新荣, 张景光, 王新平, 赵金龙, 陈应武. 用Minirhizotrons观测柠条根系生长动态[J]. 植物生态学报, 2006, 30(3): 457-464. |
| [12] | 马玉平, 王石立, 张黎, 侯英雨. 基于遥感信息的作物模型重新初始化/参数化方法研究初探[J]. 植物生态学报, 2005, 29(6): 918-926. |
| [13] | 许振柱, 周广胜, 肖春旺, 王玉辉. CO2浓度倍增和土壤干旱对两种幼龄沙生灌木碳分配的影响[J]. 植物生态学报, 2005, 29(2): 281-288. |
| [14] | 唐海萍, 史培军, 李自珍. 沙坡头地区不同配置格局油蒿和柠条水分生态位适宜度研究[J]. 植物生态学报, 2001, 25(1): 6-10. |
| [15] | 王孟本, 李洪建, 柴宝峰. 柠条(Caragana korshinskii)的水分生理生态学特性[J]. 植物生态学报, 1996, 20(6): 494-501. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19