

植物生态学报 ›› 2024, Vol. 48 ›› Issue (7): 930-942.DOI: 10.17521/cjpe.2022.0406 cstr: 32100.14.cjpe.2022.0406
收稿日期:2022-10-13
接受日期:2023-10-09
出版日期:2024-07-20
发布日期:2023-10-10
通讯作者:
* 秦伟(基金资助:
JIAO Hui-Ying, LIU Li-Qiang, YANG Jia-Xin, QIN Wei*( ), WANG Rui-Zhe
), WANG Rui-Zhe
Received:2022-10-13
Accepted:2023-10-09
Online:2024-07-20
Published:2023-10-10
Contact:
* QIN Wei(Supported by:摘要:
新疆野苹果(Malus sieversii)根际固氮菌(NFB)、解磷菌(PSB)及解钾菌(KSB)的定植数量, 不仅影响植物补充养分, 促进生长, 也可以诱导系统抗性(ISR), 提高抗性抑制疾病。因此, 探讨新疆野苹果根际NFB、PSB及KSB定植数量对生理指标的影响, 确定野苹果生长较好时根际微生物数量, 对研究其氮、磷、钾的吸收至关重要。该研究以新疆8个自然种群新疆野苹果根际土壤及植株叶片为实验材料, 分离NFB、有机解磷菌(oPSB)、无机解磷菌(iPSB)及KSB, 估算功能菌株数量并测定新疆野苹果叶片生理指标, 叶片及土壤中氮、磷、钾含量。结果表明: (1) 8个种群新疆野苹果根际4种功能菌株定植总数量存在极显著差异, 其中新源县野果林种群oPSB、iPSB、NFB及4种功能菌株定植总数最多, 巩留县那孜工队种群KSB定植数量最多; (2) 4种功能菌株总数、PSB数量与新疆野苹果叶片过氧化氢酶活性呈正相关关系, KSB数量与其叶片氧化还原酶活性呈正相关关系。证明新疆野苹果根际氮、磷、钾活化菌定植数量与其抗性存在关联。(3)在营养生长方面, oPSB数量反映叶片磷和氮含量; iPSB数量反映土壤磷含量, oPSB和NFB数量反映土壤氮含量; KSB数量反映叶片和土壤钾的含量; (4)定植数量分别在7.08 × 104 CFU·g-1 (NFB)、2.7 × 107 CFU·g-1 (PSB)及4.98 × 105 CFU·g-1 (KSB)时, 叶片及土壤中氮、磷、钾含量较高, 新疆野苹果营养生长最佳。研究确定了野苹果生长较好时根际微生物数量, 以期为施用功能菌株的接种量提供理论依据, 也为新疆野苹果林生态保护提供参考。
焦荟颖, 刘立强, 杨佳鑫, 秦伟, 王睿哲. 新疆野苹果自然种群根际固氮菌、解磷菌及解钾菌对叶片养分和生理指标的影响. 植物生态学报, 2024, 48(7): 930-942. DOI: 10.17521/cjpe.2022.0406
JIAO Hui-Ying, LIU Li-Qiang, YANG Jia-Xin, QIN Wei, WANG Rui-Zhe. Effects of rhizosphere nitrogen-fixing, phosphate-solubilizing and potassium-solubilizing bacteria on leaf nutrients and physiological traits in different natural populations of Malus sieversii. Chinese Journal of Plant Ecology, 2024, 48(7): 930-942. DOI: 10.17521/cjpe.2022.0406
| 采样地点 Sampling site | 土壤类型 Soil type | 经度 Longitude (E) | 纬度 Latitude (N) | 海拔 Altitude (m) |
|---|---|---|---|---|
| A1 | 黑钙土 Chernozem | 80.79° | 44.43° | 1 121.95 |
| A2 | 80.80° | 44.43° | 1 121.85 | |
| A3 | 80.80° | 44.44° | 1 121.92 | |
| B1 | 灰钙土 Sierozem | 81.39° | 43.93° | 625.80 |
| B2 | 81.39° | 43.93° | 625.44 | |
| B3 | 81.39° | 43.93° | 625.97 | |
| C1 | 黑钙土 Chernozem | 83.61° | 43.38° | 1 445.31 |
| C2 | 83.60° | 43.37° | 1 445.28 | |
| C3 | 83.61° | 43.38° | 1 445.32 | |
| D1 | 黑钙土 Chernozem | 82.73° | 43.20° | 1 296.37 |
| D2 | 82.73° | 43.20° | 1 296.29 | |
| D3 | 82.73° | 43.20° | 1 296.56 | |
| E1 | 棕钙土 Brown calcium soil | 82.75° | 43.22° | 1 273.59 |
| E2 | 82.75° | 43.22° | 1 273.28 | |
| E3 | 82.75° | 43.22° | 1 272.98 | |
| F1 | 黑钙土 Chernozem | 82.86° | 43.26° | 1 289.57 |
| F2 | 82.86° | 43.26° | 1 288.86 | |
| F3 | 82.86° | 43.26° | 1 289.31 | |
| G1 | 黑钙土 Chernozem | 84.00° | 46.37° | 1 250.84 |
| G2 | 84.00° | 46.37° | 1 250.67 | |
| G3 | 84.00° | 46.37° | 1 250.29 | |
| H1 | 黑钙土 Chernozem | 83.53° | 46.15° | 900.26 |
| H2 | 83.53° | 46.15° | 900.59 | |
| H3 | 83.53° | 46.15° | 900.76 |
表1 新疆野苹果根际土壤取样点概况
Table 1 Summary of rhizosphere soil sampling sites for Malus sieversii
| 采样地点 Sampling site | 土壤类型 Soil type | 经度 Longitude (E) | 纬度 Latitude (N) | 海拔 Altitude (m) |
|---|---|---|---|---|
| A1 | 黑钙土 Chernozem | 80.79° | 44.43° | 1 121.95 |
| A2 | 80.80° | 44.43° | 1 121.85 | |
| A3 | 80.80° | 44.44° | 1 121.92 | |
| B1 | 灰钙土 Sierozem | 81.39° | 43.93° | 625.80 |
| B2 | 81.39° | 43.93° | 625.44 | |
| B3 | 81.39° | 43.93° | 625.97 | |
| C1 | 黑钙土 Chernozem | 83.61° | 43.38° | 1 445.31 |
| C2 | 83.60° | 43.37° | 1 445.28 | |
| C3 | 83.61° | 43.38° | 1 445.32 | |
| D1 | 黑钙土 Chernozem | 82.73° | 43.20° | 1 296.37 |
| D2 | 82.73° | 43.20° | 1 296.29 | |
| D3 | 82.73° | 43.20° | 1 296.56 | |
| E1 | 棕钙土 Brown calcium soil | 82.75° | 43.22° | 1 273.59 |
| E2 | 82.75° | 43.22° | 1 273.28 | |
| E3 | 82.75° | 43.22° | 1 272.98 | |
| F1 | 黑钙土 Chernozem | 82.86° | 43.26° | 1 289.57 |
| F2 | 82.86° | 43.26° | 1 288.86 | |
| F3 | 82.86° | 43.26° | 1 289.31 | |
| G1 | 黑钙土 Chernozem | 84.00° | 46.37° | 1 250.84 |
| G2 | 84.00° | 46.37° | 1 250.67 | |
| G3 | 84.00° | 46.37° | 1 250.29 | |
| H1 | 黑钙土 Chernozem | 83.53° | 46.15° | 900.26 |
| H2 | 83.53° | 46.15° | 900.59 | |
| H3 | 83.53° | 46.15° | 900.76 |
| 种群 Population | 有机解磷菌 oPSB (CFU·g-1) | 无机解磷菌 iPSB (CFU·g-1) | 固氮菌 NFB (CFU·g-1) | 解钾菌 KSB (CFU·g-1) | 4种功能菌株总数 Total number of 4 functional bacterias (CFU·g-1) |
|---|---|---|---|---|---|
| A | 7.12 × 106Cc | 7.05 × 104Ef | 7.08 × 104Bb | 1.12 × 103Ccd | 7.27 × 106Cc |
| B | 1.30 × 106Ff | 3.85 × 105Cc | 3.82 × 102Gg | 1.29 × 104Bb | 1.69 × 106Ff |
| C | 2.48 × 107Aa | 2.47 × 106Aa | 8.30 × 104Aa | 5.27 × 103Cc | 2.75 × 107Aa |
| D | 3.68 × 106Dd | 3.62 × 105Cd | 1.63 × 103Ff | 1.22 × 104Bb | 4.05 × 106Dd |
| E | 8.27 × 106Bb | 8.15 × 105Bb | 8.21 × 102FGg | 5.15 × 102Cd | 9.09 × 106Bb |
| F | 2.32 × 106Ee | 5.00 × 104Ef | 5.00 × 104Cc | 4.98 × 105Aa | 2.92 × 106Ee |
| G | 7.60 × 105Gg | 1.18 × 105De | 3.54 × 104Dd | 1.19 × 102Cd | 9.13 × 105Gg |
| H | 1.65 × 104Hh | 3.72 × 103Fg | 1.23 × 104Ee | 7.94 × 102Ccd | 3.34 × 104Hh |
表2 不同种群新疆野苹果根际功能菌株定植数量
Table 2 Number of rhizosphere functional strains in different populations of Malus sieversii
| 种群 Population | 有机解磷菌 oPSB (CFU·g-1) | 无机解磷菌 iPSB (CFU·g-1) | 固氮菌 NFB (CFU·g-1) | 解钾菌 KSB (CFU·g-1) | 4种功能菌株总数 Total number of 4 functional bacterias (CFU·g-1) |
|---|---|---|---|---|---|
| A | 7.12 × 106Cc | 7.05 × 104Ef | 7.08 × 104Bb | 1.12 × 103Ccd | 7.27 × 106Cc |
| B | 1.30 × 106Ff | 3.85 × 105Cc | 3.82 × 102Gg | 1.29 × 104Bb | 1.69 × 106Ff |
| C | 2.48 × 107Aa | 2.47 × 106Aa | 8.30 × 104Aa | 5.27 × 103Cc | 2.75 × 107Aa |
| D | 3.68 × 106Dd | 3.62 × 105Cd | 1.63 × 103Ff | 1.22 × 104Bb | 4.05 × 106Dd |
| E | 8.27 × 106Bb | 8.15 × 105Bb | 8.21 × 102FGg | 5.15 × 102Cd | 9.09 × 106Bb |
| F | 2.32 × 106Ee | 5.00 × 104Ef | 5.00 × 104Cc | 4.98 × 105Aa | 2.92 × 106Ee |
| G | 7.60 × 105Gg | 1.18 × 105De | 3.54 × 104Dd | 1.19 × 102Cd | 9.13 × 105Gg |
| H | 1.65 × 104Hh | 3.72 × 103Fg | 1.23 × 104Ee | 7.94 × 102Ccd | 3.34 × 104Hh |
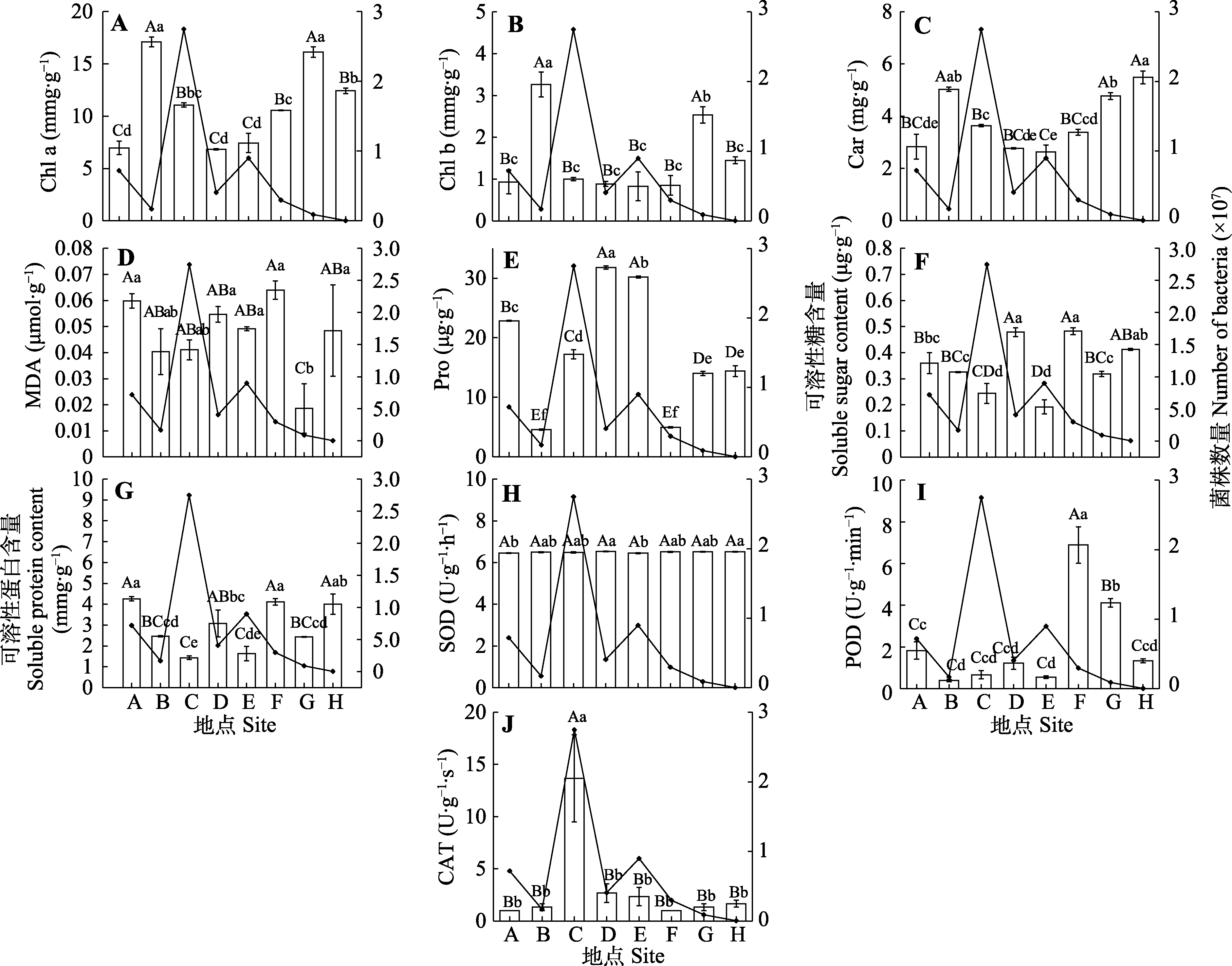
图1 不同种群新疆野苹果叶片生理指标(柱形)及其根际4种功能菌株总数量(折线)变化(平均值±标准误)。Car, 类胡萝卜素含量; CAT, 过氧化氢酶活性; Chl a, 叶绿素a含量; Chl b, 叶绿素b含量; MDA, 丙二醛含量; POD, 过氧化物酶活性; Pro, 脯氨酸含量; SOD, 超氧化物歧化酶活性。不同小写字母表示种群间在0.05水平差异显著, 不同大写字母表示在0.01水平差异显著。地点同表1。
Fig. 1 Changes in leaf physiological characteristics (column) of different populations of Malus sieversii and their total number (polyline) of four functional strains of rhizosphere bacteria (mean ± SE). Car, carotenoid content; CAT, catalase activity; Chl a, chlorophyll a content; Chl b, chlorophyll b content; MDA, malondialdehyde content; POD, peroxidase activity; Pro, proline content; SOD, superoxide dismutase activity. Distinct lowercase letters denote significant differences at the 0.05 significance level, while differing uppercase letters signify significant distinctions at the 0.01 significance level among populations. Site see Table 1.
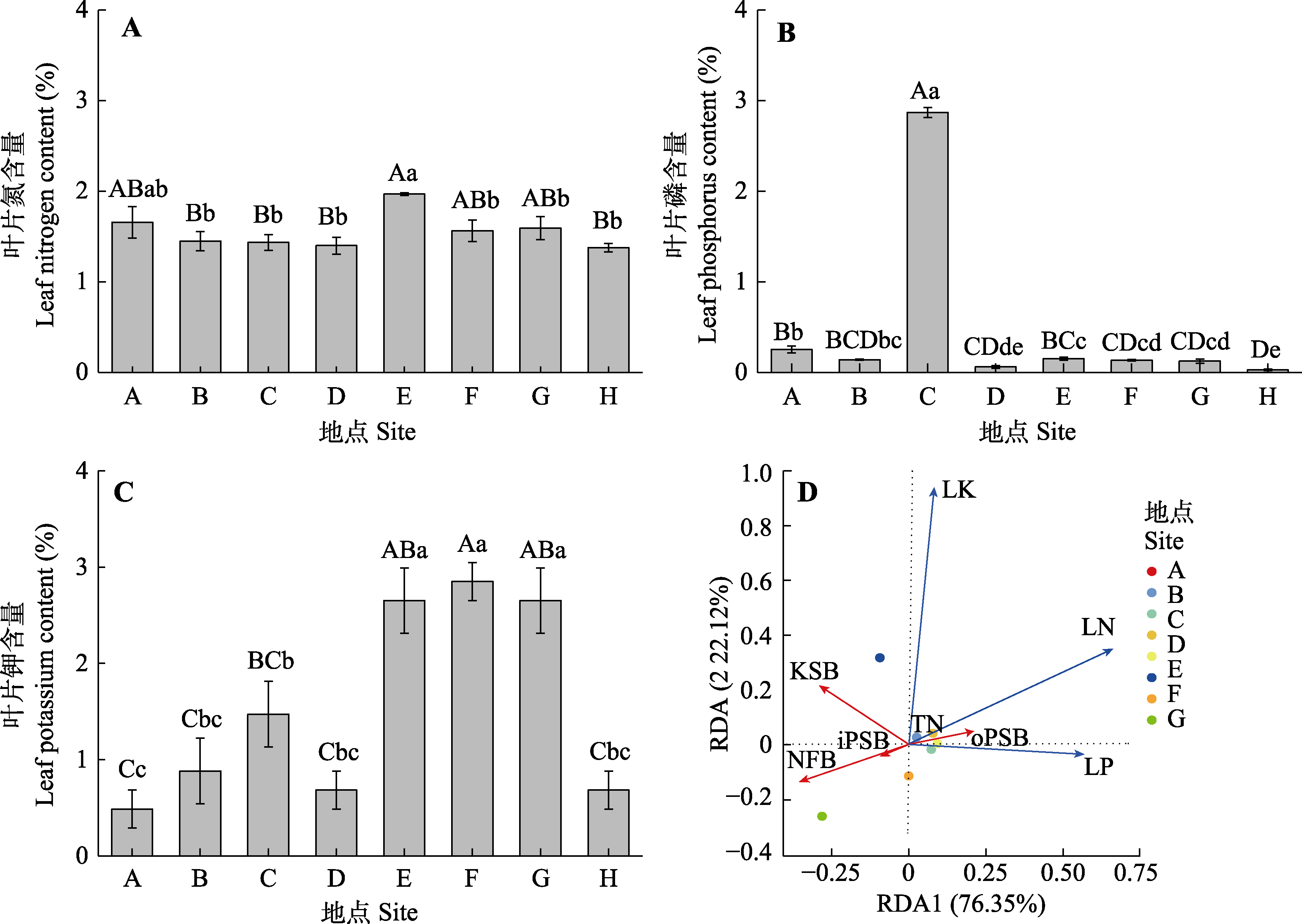
图2 不同种群新疆野苹果叶片氮磷钾含量(平均值±标准误)及其与根际NFB、PSB及KSB的数量冗余分析(RDA)。iPSB, 无机解磷菌; KSB, 解钾菌; LK, 叶片钾含量; LN, 叶片氮含量; LP, 叶片磷含量; NFB, 固氮菌; oPSB, 有机解磷菌; TN, 4种功能菌株总数。不同小写字母表示种群间在0.05水平差异显著, 不同大写字母表示在0.01水平差异显著。地点同表1。
Fig. 2 Redundancy analysis of nitrogen, phosphorus and potassium content (mean ± SE) in leaves of Malus sierversii of different populations and the number of NFB, PSB, and KSB. iPSB, inorganic phosphate-solubilizing bacteria; KSB, potassium-solubilizing bacteria; NFB, nitrogen-fixing bacteria; oPSB, organic phosphate-solubilizing bacteria; TN, total number of four functional strains. LN, leaf nitrogen content; LK, leaf potassium content; LP, leaf phosphorus content. Distinct lowercase letters denote significant differences at the 0.05 significance level, while differing uppercase letters signify significant distinctions at the 0.01 significance level among populations. Site see Table 1.
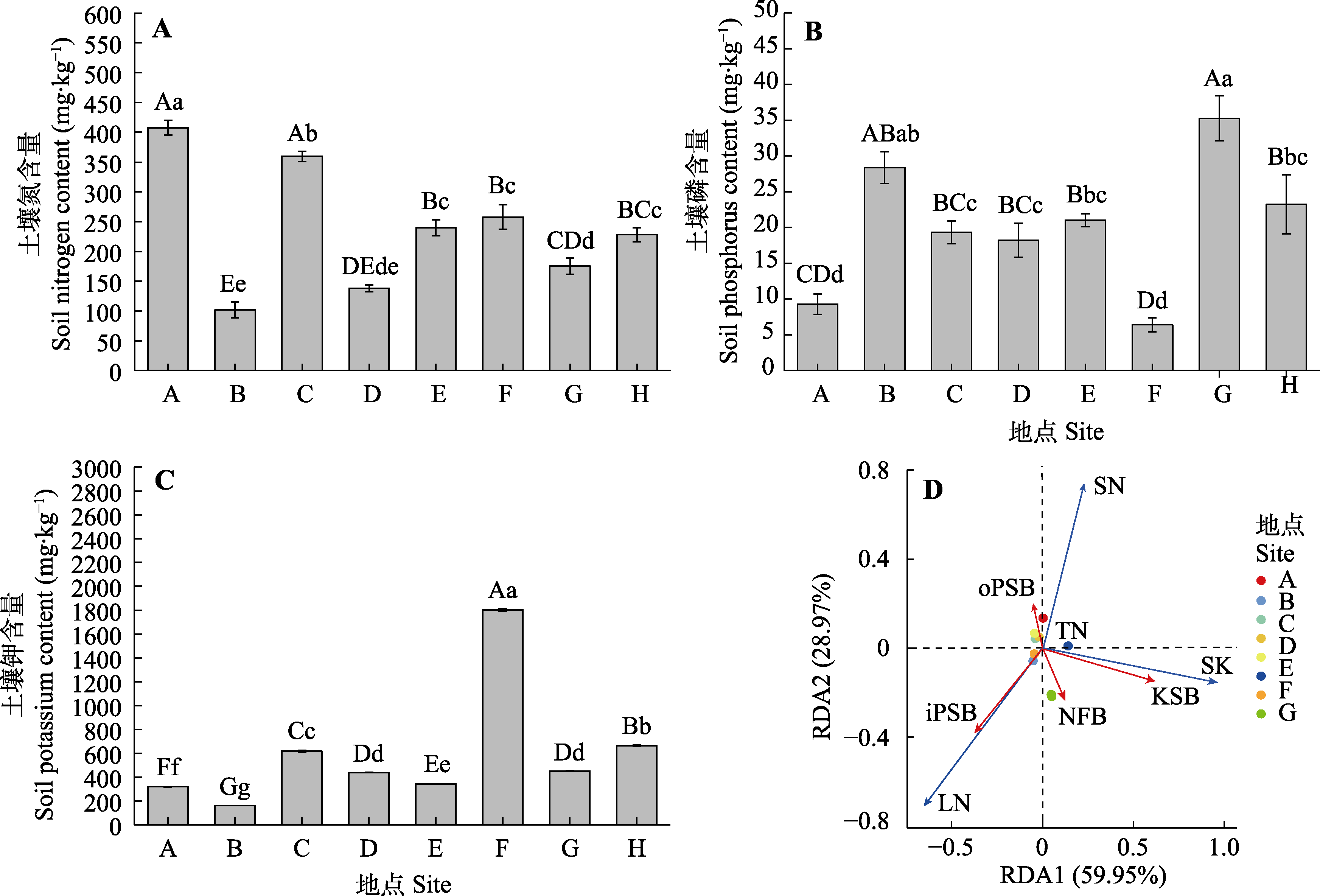
图3 不同种群新疆野苹果土壤氮磷钾含量(平均值±标准误)及其与新疆野苹果根际NFB、PSB及KSB的数量冗余分析(RDA)。iPSB, 无机解磷菌的数量; KSB, 解钾菌的数量; NFB, 固氮菌的数量; oPSB, 有机解磷菌的数量; TN, 4种功能菌株总数。SK, 土壤钾含量; SN, 土壤氮含量; SP, 土壤磷含量。不同小写字母表示种群间在0.05水平差异显著, 不同大写字母表示在0.01水平差异显著。地点同表1。
Fig. 3 Redundanly analysis analysis of nitrogen, phosphorus and potassium content (mean ± SD) in soil of different populations and their relationship with the number of NFB, PSB, and KSB in the rhizosphere of Malus sieversii in Xinjiang. iPSB, inorganic phosphate-solubilizing bacteria; KSB, potassium-solubilizing bacteria; NFB, nitrogen-fixing bacteria; oPSB, organic phosphate-solubilizing bacteria; TN, total number of four functional strains. SN, soil nitrogen content; SK, soil potassium content; SP, soil phosphorus content. Distinct lowercase letters denote significant differences at the 0.05 significance level, while differing uppercase letters signify significant distinctions at the 0.01 significance level among populations. Site see Table 1.
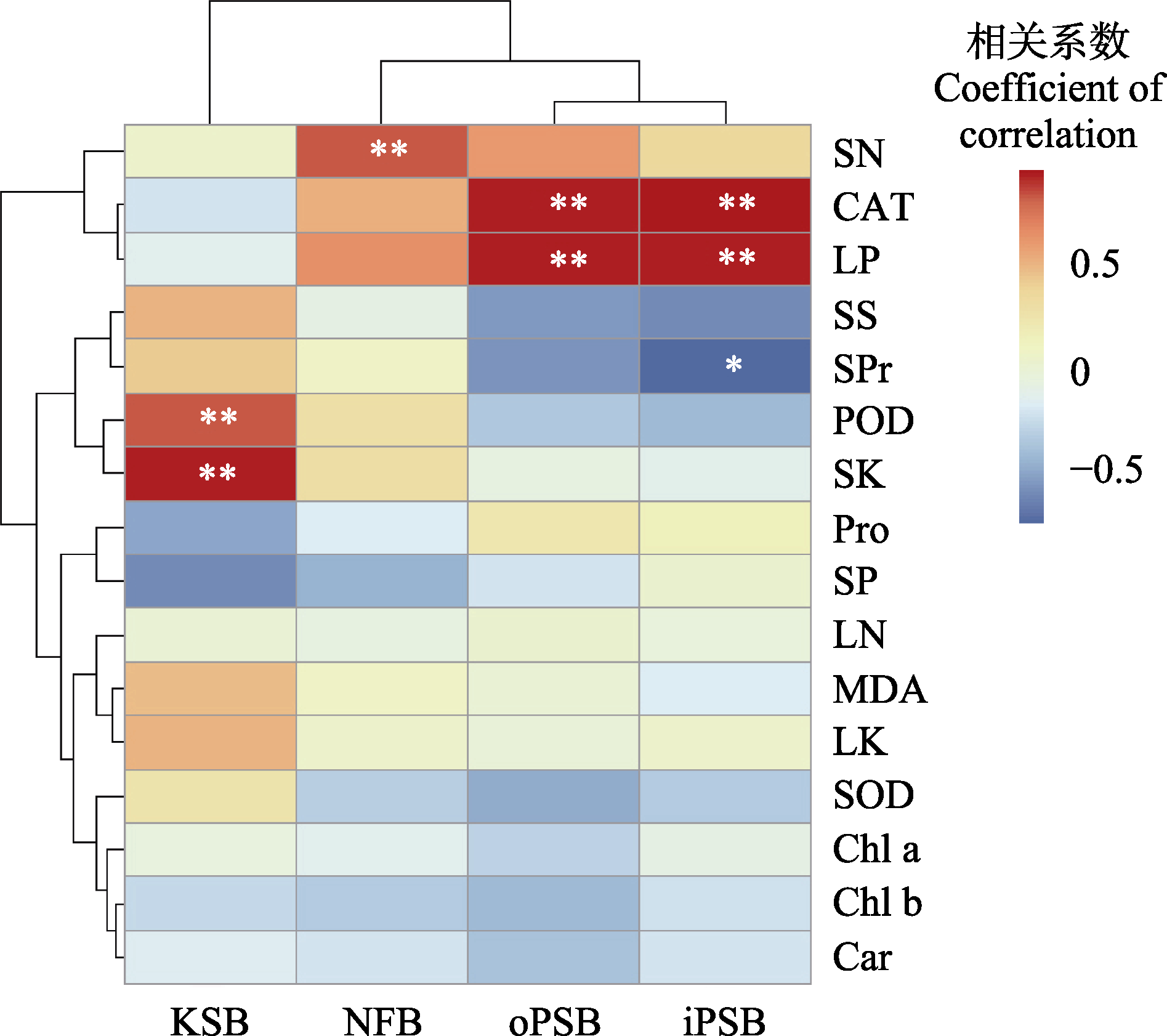
图4 新疆野苹果根际功能菌株数量与生理指标、氮磷钾含量的相关性。Car, 类胡萝卜素含量; CAT, 过氧化氢酶活性; Chl a, 叶绿素a含量; Chl b, 叶绿素b含量; iPSB, 无机解磷菌的数量; KSB, 解钾菌的数量; LN, 叶片氮含量; LK, 叶片钾含量; LP, 叶片磷含量; MDA, 丙二醛含量; NFB, 固氮菌的数量; oPSB, 有机解磷菌的数量; POD, 过氧化物酶活性; Pro, 脯氨酸含量; SK, 土壤钾含量; SN, 土壤氮含量; SOD, 超氧化物歧化酶活性; SP, 土壤磷含量; Spr, 可溶性蛋白含量; SS, 可溶性糖含量。*, p ≤ 0.05; **, p ≤ 0.01。
Fig. 4 Correlation between the population of rhizosphere functional bacteria and various physiological characteristics, as well as the nitrogen, phosphorus, and potassium content of Malus sieversii. Car, carotenoid content; CAT, catalase activity; Chl a, chlorophyll a content; Chl b, chlorophyll b content; LN, leaf nitrogen content; LK, leaf potassium content; LP, leaf phosphorus content; MDA, malondialdehyde content; POD, peroxidase activity; Pro, proline content; SK, soil potassium content; SN, soil nitrogen content; SOD, superoxide dismutase activity; SP, soil phosphorus content; Spr, soluble protein content; SS, soluble sugar content. iPSB, inorganic phosphate-solubilizing bacteria; KSB, potassium-solubilizing bacteria; NFB, nitrogen-fixing bacteria; oPSB, organic phosphate-solubilizing bacteria. *, p ≤ 0.05; **, p ≤ 0.01.
| [1] | Abdel Latef AAH, Omer AM, Badawy AA, Osman MS, Ragaey MM (2021). Strategy of salt tolerance and interactive impact of azotobacter chroococcum and/or alcaligenes faecalis inoculation on canola (Brassica napus L.) plants grown in saline soil. Plants, 10, 110. DOI: 10.3390/plants10010110. |
| [2] | Bakhshandeh E, Pirdashti H, Lendeh KS (2017). Phosphate and potassium-solubilizing bacteria effect on the growth of rice. Ecological Engineering, 103, 164-169. |
| [3] |
Camejo D, Guzman-Cedeno A, Moreno A (2016). Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiology and Biochemistry, 103, 10-23.
DOI PMID |
| [4] |
Chen JQ, Zhao GY, Wei YH, Dong YH, Hou LY, Jiao RZ (2021). Isolation and screening of multifunctional phosphate solubilizing bacteria and its growth-promoting effect on Chinese fir seedlings. Scientific Reports, 11, 9081. DOI: 10.1038/s41598-021-88635-4.
PMID |
| [5] | Chen YF, Ye JR, Kong QQ (2020a). Potassium-solubilizing activity of Bacillus aryabhattai SK1-7 and its growth-promoting effect on Populus alba L. Forests, 11, 1348. DOI: 10.3390/f11121348. |
| [6] | Chen YH, Yang XZ, Li Z, An XH, Ma RP, Li YQ (2020b). Efficiency of potassium-solubilizing Paenibacillus mucilaginosus for the growth of apple seedling. Journal of Integrative Agriculture, 19, 2458-2469. |
| [7] | Cornut I, Le Maire G, Laclau JP, Guillemot J, Mareschal L, Nouvellon Y, Delpierre N (2021). Potassium limitation of wood productivity: a review of elementary processes and ways forward to modelling illustrated by Eucalyptus plantations. Forest Ecology and Management, 494, 119275. DOI: 10.1016/j.foreco.2021.119275. |
| [8] | Cui Z, Zhang Y, Zhang X, Luo Z, Zhang P, Golec J, Poland TM, Zalucki MP, Han P, Lu Z (2019). Life history and mortality factors of Agrilus mali Matsumura (Coleoptera: Buprestidae) in wild apples in Northwestern China. Agricultural and Forest Entomology, 21, 309-317. |
| [9] | Dias S, Chambel L, Tenreiro R, Nunes L, Loureiro V (2021). Microbial characterization of yellow curing process of codfish. International Journal of Food Science, 2021, 6072731. DOI: 10.1155/2021/6072731. |
| [10] | Dong X, Lv L, Wang WJ, Liu YZ, Yin, CH, Xu QQ, Yan H, Fu JX, Liu XL (2019). Differences in distribution of potassium-solubilizing bacteria in forest and plantation soils in Myanmar. International Journal of Environmental Research and Public Health, 16, 700. DOI: 10.3390/ijerph16050700. |
| [11] |
Duan N, Bai Y, Sun H, Wang N, Ma Y, Li M, Wang X, Jiao C, Legall N, Mao L, Wan S, Wang K, He T, Feng S, Zhang Z, et al. (2017). Genome re-sequencing reveals the history of apple and supports a two-stage model for fruit enlargement. Nature Communications, 8, 249. DOI: 10.1038/s41467-017-00336-7.
PMID |
| [12] | Duan YN, Chen R, Zhang R, Jiang WT, Chen XS, Yin CM, Mao ZQ (2022). Isolation and identification of Bacillus vallismortis HSB-2 and its biocontrol potential against apple replant disease. Biological Control, 170, 104921. DOI: 10.1016/j.biocontrol.2022.104921. |
| [13] | Etesami H, Emami S, Alikhani HA (2017). Potassium solubilizing bacteria (KSB): mechanisms, promotion of plant growth, and future prospects—A review. Journal of Soil Science and Plant Nutrition, 17, 897-911. |
| [14] | Fahsi N, Mahdi I, Mesfioui A, Biskri L, Allaoui A (2021). Plant growth-promoting rhizobacteria isolated from the jujube (Ziziphus lotus) plant enhance wheat growth, Zn uptake, and heavy metal tolerance. Agriculture, 11, 316. DOI: 10.3390/agriculture11040316. |
| [15] |
Gamez R, Cardinale M, Montes M, Ramirez S, Schnell S, Rodriguez F (2019). Screening, plant growth promotion and root colonization pattern of two rhizobacteria (Pseudomonas fluorescens Ps006 and Bacillus amyloliquefaciens Bs006) on banana cv. Williams (Musa acuminata Colla). Microbiological Research, 220, 12-20.
DOI PMID |
| [16] | García-Carmona M, García-Orenes F, Mataix-Solera J, Roldán A, Pereg L, Caravaca F (2021). Salvage logging alters microbial community structure and functioning after a wildfire in a Mediterranean forest. Applied Soil Ecology, 168, 104130. DOI: 10.1016/j.apsoil.2021.104130. |
| [17] | Grover M, Bodhankar S, Sharma A, Sharma P, Singh J, Nain L (2021). PGPR mediated alterations in root traits: way toward sustainable crop production. Frontiers in Sustainable Food Systems, 4, 618230. DOI: 10.3389/fsufs.2020.618230. |
| [18] | Ha YH, Oh SH, Lee SR (2021). Genetic admixture in the population of wild apple (Malus sieversii) from the Tien Shan Mountains, Kazakhstan. Genes, 12, 104. DOI: 10.3390/genes12010104. |
| [19] | Ju I, Wj B, Sila MD, Onyimba IA, Egbere OJ (2018). A review: Biofertilizer—A key player in enhancing soil fertility and crop productivity. Journal of Experimental and Clinical Microbiology, 2, 22-28. |
| [20] | Khan M, Imran QM, Shahid M, Mun BG, Lee SU, Khan MA, Hussain A, Lee TJ, Yun BW (2019). Nitric oxide-induced AtAO (3) differentially regulates plant defense and drought tolerance in Arabidopsis thaliana. BMC Plant Biology, 19, 602. DOI: 10.1186/s12870-019-2210-3. |
| [21] | Kour D, Rana KL, Kaur T, Yadav N, Yadav AN, Kumar M, Kumar V, Dhaliwal HS, Saxena AK (2021). Biodiversity, current developments and potential biotechnological applications of phosphorus-solubilizing and -mobilizing microbes: a review. Pedosphere, 31, 43-75. |
| [22] | Kour D, Rana KL, Yadav AN, Yadav N, Kumar M, Kumar V, Vyas P, Dhaliwal HS, Saxena AK (2020). Microbial biofertilizers: bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatalysis and Agricultural Biotechnology, 23, 101487. DOI: 10.1016/j.bcab.2019.101487. |
| [23] | Lee Díaz AS, Macheda D, Saha H, Ploll U, Orine D, Biere A (2021). Tackling the context-dependency of microbial-induced resistance. Agronomy, 11, 1293. DOI: 10.3390/agronomy11071293. |
| [24] | Li D, Bodjrenou DM, Zhang S, Wang B, Pan H, Yeh KW, Lai Z, Cheng C (2021). The endophytic fungus Piriformospora indica reprograms banana to cold resistance. International Journal of Molecular Sciences, 22, 4973. DOI: 10.3390/ijms22094973. |
| [25] | Li JQ, Meng B, Chai H, Yang XC, Song WZ, Li SX, Lu A, Zhang T, Sun W (2019). Arbuscular mycorrhizal fungi alleviate drought stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) grasses via altering antioxidant enzyme activities and photosynthesis. Frontiers in Plant Science, 10, 499. DOI: 10.3389/fpls.2019.00499. |
| [26] | Lo’ay AA, El-Ezz SFA, Awadeen AA (2021). Effect of different foliar potassium fertilization forms on vegetative growth, yield, and fruit quality of kaki trees grown in sandy soil. Scientia Horticulturae, 288, 110420. DOI: 10.1016/j.scienta.2021.110420. |
| [27] | Loon LC (2007). Plant responses to plant growth-promoting rhizobacteria. European Journal of Plant Pathology, 119, 243-254. |
| [28] | Meena VS, Maurya BR, Verma JP, Aeron A, Kumar A, Kim K, Bajpai VK (2015). Potassium solubilizing rhizobacteria (KSR): isolation, identification, and K-release dynamics from waste mica. Ecological Engineering, 81, 340-347. |
| [29] |
Mehta P, Walia A, Chauhan A, Shirkot CK (2013). Plant growth promoting traits of phosphate-solubilizing rhizobacteria isolated from apple trees in trans Himalayan region of Himachal Pradesh. Archives of Microbiology, 195, 357-369.
DOI PMID |
| [30] |
Mikiciuk G, Sas-Paszt L, Mikiciuk M, Derkowska E, Trzcinski P, Gluszek S, Lisek A, Wera-Bryl S, Rudnicka J (2019). Mycorrhizal frequency, physiological parameters, and yield of strawberry plants inoculated with endomycorrhizal fungi and rhizosphere bacteria. Mycorrhiza, 29, 489-501.
DOI PMID |
| [31] | Mise K, Koyama Y, Matsumoto A, Fujita K, Kunito T, Senoo K, Otsuka S (2020). Pectin drives microbial phosphorus solubilization in soil: evidence from isolation-based and community-scale approaches. European Journal of Soil Biology, 97, 103169. DOI: 10.1016/j.ejsobi.2020.103169. |
| [32] | Murphy J, Riley JP (1962). A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta, 27, 31-36. |
| [33] | Nacoon S, Jogloy S, Riddech N, Mongkolthanaruk W, Kuyper TW, Boonlue S (2020). Interaction between phosphate solubilizing bacteria and arbuscular mycorrhizal fungi on growth promotion and tuber inulin content of Helianthus tuberosus L. Scientific Reports, 10, 4916. DOI: 10.1038/s41598-020-61846-x. |
| [34] | Ren LX, Wang BS, Yue CP, Zhou SP, Zhang SH, Huo HW, Xu GH (2019). Mechanism of application nursery cultivation arbuscular mycorrhizal seedling in watermelon in the field. Annals of Applied Biology, 174, 51-60. |
| [35] | Rudack K, Seddig S, Sprenger H, Köhl K, Uptmoor R, Ordon F (2017). Drought stress-induced changes in starch yield and physiological traits in potato. Journal of Agronomy and Crop Science, 203, 494-505. |
| [36] |
Rudrappa T, Biedrzycki ML, Bais HP (2008). Causes and consequences of plant-associated biofilms. FEMS Microbiology Ecology, 64, 153-166.
DOI PMID |
| [37] | Saadati S, Baninasab B, Mobli M, Gholami M (2020). Cold tolerance in olive leaves of three cultivars related to some physiological parameters during cold acclimation and de-acclimation stages. Journal of Agricultural Science and Technology, 22, 1313-1326. |
| [38] | Santana EB, Dias JCT (2019). Phosphate solubilizing activity of native soil microorganisms from the rhizosphere of Jatropha curcas and from phosphate-solubilizing bacteria inoculum. Genetics and Molecular Research, 18, gmr18271. DOI: 10.4238/gmr18271. |
| [39] | Santoyo G, Urtis-Flores CA, Loeza-Lara PD, Orozco-Mosqueda MDC, Glick BR (2021). Rhizosphere colonization determinants by plant growth-promoting rhizobacteria (PGPR). Biology, 10, 475. DOI: 10.3390/biology10060475. |
| [40] | Shan QJ, Wang ZK, Ling HB, Zhang GP, Yan, JJ, Han FF (2021). Unreasonable human disturbance shifts the positive effect of climate change on tree-ring growth of Malus sieversii in the origin area of world cultivated apples. Journal of Cleaner Production, 287, 125008. DOI: 10.1016/j.jclepro.2020.125008. |
| [41] | Silva BN, Picanco BM, Hawerroth C, Silva LC, Rodrigues FA (2022). Physiological and biochemical insights into induced resistance on tomato against septoria leaf spot by a phosphite combined with free amino acids. Physiological and Molecular Plant Pathology, 120, 101854. DOI: 10.1016/j.pmpp.2022.101854. |
| [42] | Simranjit K, Kanchan A, Prasanna R, Ranjan K, Ramakrishnan B, Singh AK, Shivay YS (2019). Microbial inoculants as plant growth stimulating and soil nutrient availability enhancing options for cucumber under protected cultivation. World Journal of Microbiology Biotechnology, 35, 1-14. |
| [43] | Singh SR, Zargar MY, Najar GR, Peer FA, Ishaq M (2013). Microbial dynamics, root colonization, and nutrient availability as influenced by inoculation of liquid bioinoculants in cultivars of apple seedlings. Communications in Soil Science and Plant Analysis, 44, 1511-1523. |
| [44] | Spengler RN (2019). Origins of the apple: the role of megafaunal mutualism in the domestication of malus and rosaceous trees. Frontiers in Plant Science, 10, 617. DOI: 10.3389/fpls.2019.00617. |
| [45] | Sun F, Ou QJ, Wang N, Guo ZX, Ou YY, Li N, Peng CL (2020). Isolation and identification of potassium-solubilizing bacteria from Mikania micrantha rhizospheric soil and their effect on M. micrantha plants. Global Ecology and Conservation, 23, e01141. DOI: 10.1016/j.gecco.2020.e01141. |
| [46] | Tinna D, Garg N, Sharma S, Pandove G, Chawla N (2020). Utilization of plant growth promoting rhizobacteria as root dipping of seedlings for improving bulb yield and curtailing mineral fertilizer use in onion under field conditions. Scientia Horticulturae, 270, 109432. DOI: 10.1016/j.scienta.2020.109432. |
| [47] | Varma A, Tripathi S, Prasad R (2019). Plant Biotic Interactions. Springer, Cham, Switzerland. |
| [48] | Wisniewski M, Artlip T, Liu J, Ma J, Burchard E, Norelli J, Dardick C (2020). Fox hunting in wild apples: searching for novel genes in Malus sieversii. International Journal of Molecular Sciences, 21, 9516. DOI: 10.3390/ijms21249516. |
| [49] | Xie H, Liu RY, Xu YX, Liu X, Sun FX, Ma YH, Wang YY (2022). Effect of in situ bioremediation of soil contaminated with DDT and DDE by Stenotrophomonas sp. strain DXZ9 and ryegrass on soil microorganism. Microbiology Research, 13, 64-86. |
| [50] | Zhang H, Sun XP, Dai MQ (2022). Improving crop drought resistance with plant growth regulators and rhizobacteria: Mechanisms, applications, and perspectives. Plant Communications, 3, 100228. DOI: 10.1016/j.xplc.2021.100228. |
| [51] | Zhang HX, Zhang ML, Wang LN (2014). Genetic structure and historical demography of Malus sieversii in the Yili Valley and the western mountains of the Junggar Basin, Xinjiang, China. Journal of Arid Land, 7, 264-271. |
| [52] | Zhang JT, Mu CS (2009). Effects of saline and alkaline stresses on the germination, growth, photosynthesis, ionic balance and anti-oxidant system in an alkali-tolerant leguminous forage Lathyrus quinquenervius. Soil Science and Plant Nutrition, 55, 685-697. |
| [53] | Zhang P, Lyu ZZ, Zhang X, Zhao XP, Zhang YG, Tanabekova G, Bagila M, Zhanera A, Cui ZJ (2019). Age structure of Malus sieversii (Ledeb.) Roem. population in Ili of Xinjiang and Kazakhstan. Arid Zone Research, 36, 844-853. |
| [张萍, 吕昭智, 张鑫, 赵想平, 张永光, Tanabekova G, Bagila M, Zhanera A, 崔志军 (2019). 新疆伊犁与哈萨克斯坦新疆野苹果(Malus sieversii (Ledeb.) Roem.)种群年龄结构. 干旱区研究, 36, 844-853.] |
| [1] | 罗源林, 马文红, 张芯毓, 苏闯, 史亚博, 赵利清. 内蒙古锦鸡儿属植物地理替代分布种的功能性状沿环境梯度的变化[J]. 植物生态学报, 2022, 46(11): 1364-1375. |
| [2] | 武运涛, 杨森, 王欣, 黄俊胜, 王斌, 刘卫星, 刘玲莉. 草地土壤有机质不同组分氮库对长期氮添加的响应[J]. 植物生态学报, 2021, 45(7): 790-798. |
| [3] | 于青含, 金光泽, 刘志理. 植株大小、枝龄和环境共同驱动红松枝性状的变异[J]. 植物生态学报, 2020, 44(9): 939-950. |
| [4] | 庞芳, 夏维康, 何敏, 祁珊珊, 戴志聪, 杜道林. 固氮菌缓解氮限制环境中丛枝菌根真菌对加拿大一枝黄花的营养竞争[J]. 植物生态学报, 2020, 44(7): 782-790. |
| [5] | 闫雅楠, 叶小齐, 吴明, 闫明, 张昕丽. 入侵植物加拿大一枝黄花根际解钾菌多样性及解钾活性[J]. 植物生态学报, 2019, 43(6): 543-556. |
| [6] | 冯婵莹, 郑成洋, 田地. 氮添加对森林植物磷含量的影响及其机制[J]. 植物生态学报, 2019, 43(3): 185-196. |
| [7] | 张静, 刘耘华, 盛建东, 柴强, 李瑞霞, 赵丹. 新疆北部草地典型灌木的碳氮特征[J]. 植物生态学报, 2018, 42(3): 307-316. |
| [8] | 颉洪涛, 虞木奎, 成向荣. 光照强度变化对5种耐阴植物氮磷养分含量、分配以及限制状况的影响[J]. 植物生态学报, 2017, 41(5): 559-569. |
| [9] | 司晓林, 王文银, 高小刚, 徐当会. 氮硅添加对高寒草甸垂穗披碱草叶片全氮含量及净光合速率的影响[J]. 植物生态学报, 2016, 40(12): 1238-1244. |
| [10] | 王乔姝怡, 郑成洋, 张歆阳, 曾发旭, 邢娟. 氮添加对武夷山亚热带常绿阔叶林植物叶片氮磷化学计量特征的影响[J]. 植物生态学报, 2016, 40(11): 1124-1135. |
| [11] | 王常顺, 汪诗平. 植物叶片性状对气候变化的响应研究进展[J]. 植物生态学报, 2015, 39(2): 206-216. |
| [12] | 吴浩, 卢志军, 黄汉东, 江明喜. 三种植物对土壤磷吸收和富集能力的比较[J]. 植物生态学报, 2015, 39(1): 63-71. |
| [13] | 杨浩, 罗亚晨. 糙隐子草功能性状对氮添加和干旱的响应[J]. 植物生态学报, 2015, 39(1): 32-42. |
| [14] | 闫霜,张黎,景元书,何洪林,于贵瑞. 植物叶片最大羧化速率与叶氮含量关系的变异性[J]. 植物生态学报, 2014, 38(6): 640-652. |
| [15] | 张林, 阎恩荣, 魏海霞, 刘新圣, 沈维. 藏东南色季拉山林线过渡带7种灌木植物的叶氮回收潜力[J]. 植物生态学报, 2014, 38(12): 1325-1332. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19